Which Element Has the Following Configuration Xe 6s24f75d1
By convention they are written last in noble gas. We review their content and use your feedback to keep the quality high.
Which Element Has The Following Configuration Math 54 Xe 6s 24f 5 Math Quora
I know its Ce but i need the electrons number too.

. An element that has the valence electron configuration 3s23p3 belongs to which period and group. Z5421410282 and therefore the element is LEAD. Start your trial now.
Which ion has the smallest ionic radius. Be sure your answer has a unit symbol if necessary and round it to 4 significant digițs. Experts are tested by Chegg as specialists in their subject area.
In the below periodic table you can see the trend of Electron Configuration. View Available Hint s. Which ion has the same e- configuration as Kr.
100 18 ratings An alternative way to write the electron conf. Which element has the following configuration Xe6s24f75d1. Which element has the following configuration.
An element that has 3 electrons in the n 2 energy level. This element is a halogen. What is the ground state electron configuration for the ion Hg2 Xe4f145d106s2.
Of the following which atom has the largest atomic radius. This problem has been solved. We review their content and use your feedback to keep the quality high.
Who are the experts. O x10 I Explanation Check II. Of the following which element has the highest first ionization energy.
Which element has the following configuration. View the full answer. Xe6s 2 4f 3.
Value is a guess based on periodic table trend. Xe 6s2 4f1 5d1There are a total of 58 electrons. Enter the chemical symbol for the element.
Enter the chemical symbol for the element. This formula says that lead has all the electrons of xenon as well as the electrons listed after Xe. Enter the chemical symbol for the element.
Math Chemistry Biology Programming Arts History BusinessLanguage Spanish EnglishTipsReviewBlog Home Which element has the following configuration 6s2 4f7 5d1 June 27 2021June 27 2021 thanh Which element has the following configuration 6s24f75d1. A Silver b Gold c Copper d Paladium. The element belongs to d-block of the periodic table and it will be a transition metal.
The given element has electronic configuration. Which element has the following electron configuration Xe 4f14 5d10 6s1. The electronic configuration ends as 2 electrons in s orbital 14 electrons in f orbital and 7 electrons in d orbital.
How many electrons are in the outermost shell of the ln3 ion in its ground state. The element lead has the electron configuration Xe 4f14 5d10 6s2 6p2. See the answer See the answer done loading.
We review their content and use your feedback to keep the quality high. Chemistry questions and answers. The electronic configuration is there to distract you.
Which element fits the following description. O CHEMICAL REACTIONS Interconverting number of atoms and mass of compound Calculate the mass of tetraborane BH10 that contains a trillion 1000 102 hydrogen atoms. D all of the above.
Solution for Which element has the following electron configuration Xe 4f14 5d10 6s1. Which element has the following configuration. The element is defined by Z the atomic number which is the number of protons positively charged massive nuclear particles.
A Silver b Gold c Copper d Paladium. You have been given the number of electrons which for the neutral element is necessarily the same as the number of. Who are the experts.
Astatine is the element that has the atomic number of 85 on the periodic table. For facts physical properties chemical properties structure and atomic properties of the specific element click. Which element has the following configuration.
This is the best answer based on feedback and ratings. The electron configuration for astatine is Xe 4f14 5d10 6s2 6p5. Of the following which element has the highest first ionization energy.
Which element has the ground state electron configuration Xe6s24f75d1. Which element has the following configuration. Which element has the ground state electon configuration Xe6s24f75d1.
Experts are tested by Chegg as specialists in their subject area. View the full answer. How many UNPAIRED electrons are in the ground state electron configuration for carbon.
Rn7s 2 5f 14 6d 10 7p 5 note Praseodymium. Periodic Table of Elements with Electron Configuration Trends. See the answer See the answer done loading.
The 6s and 6p electrons are in the outermost shell and are therefore the valence electrons. So the last electron comes in d orbital. Rn7s 2 5f 14 6d 10 7p 6 note Notes on the Electron Configuration of particular elements.
First week only 499. According to Madelung rule which describes the electronic configuration and filling up of el. Part C Which element has the following configuration.
A Rb b Br-c Se2-d all of the above. The chemical symbol of the element is Cerium ie. Of the following which element has the highest first ionization energy.
Xe has 54 electron 6s orbital has 2 electron and 4f orbital has 5 electron together 5425 61 the element with atomic number 61 is Promethium Pm which is a Lanthanide. Simple way to get to this answer is to count the number of electron or protons in the given configuration. Or the atomic number is 58 and the element is Cerium.
The metal is Iridium. What is the quantum of light called. Experts are tested by Chegg as specialists in their subject area.
A aluminum b magnesium c silicon d sodium.

Transition Elements And Coordination Chemistry Ppt Download
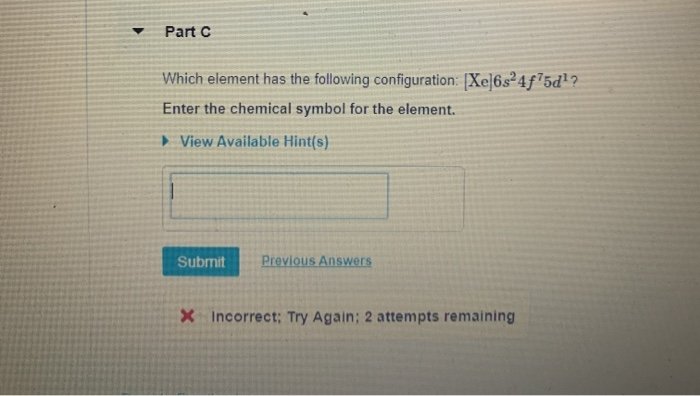
Solved Part Which Element Has The Following Configuration Chegg Com
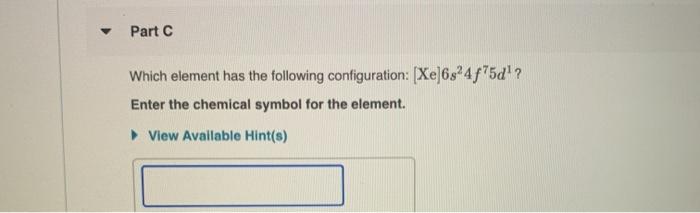
Solved Part C Which Element Has The Following Configuration Chegg Com

No comments for "Which Element Has the Following Configuration Xe 6s24f75d1"
Post a Comment